Introduction:
For the production of biotechnological products, expression of therapeutic proteins in E.coli cells is commonly used. Due to which there is a possibility of DNA contamination from the host cells in the products which are manufactured. However, the limit of DNA contamination from the cell lines has been set by regulatory agencies. According to WHO the limit of DNA contamination should not exceed 10 ng/dose in the products manufactured. One of the most common method which is widely used for detection of host cell DNA contamination by biopharmaceutical manufacturers is the PicoGreenP TMP Dye based assay. The research and development department at Krishgen Biosystems has designed a kit to detect and quantify host cell DNA in the products manufactured by recombinant expression in E.coli cell lines based on PicoGreenP TMP Dye assay.
Principle:
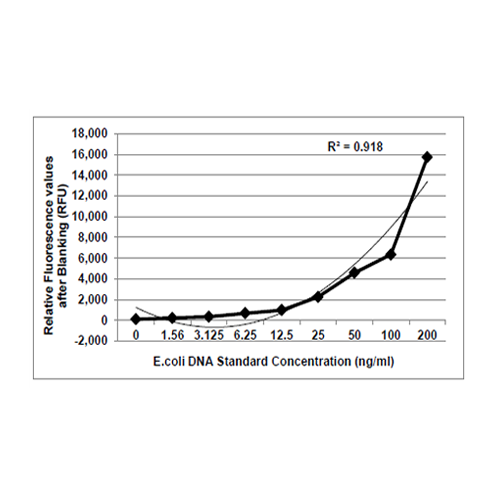
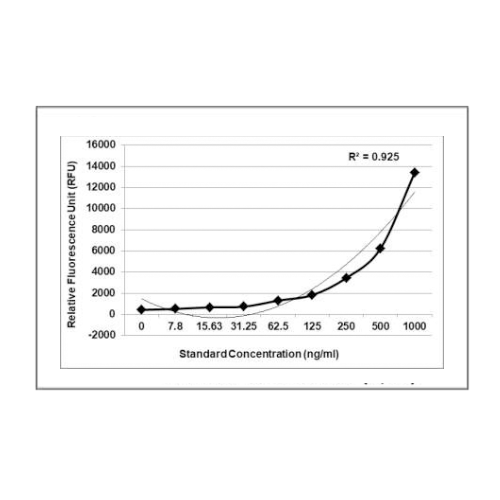
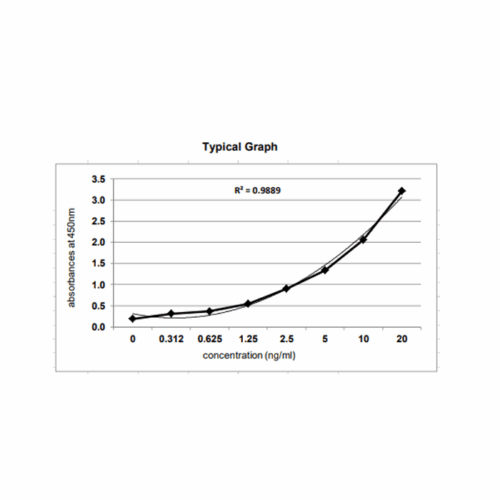
The Krishgen E.coli Host Cell DNA Kit is based on the DNA dye binding assay which utilizes PicoGreenP TM Pdye. The DNA samples and the standards are reacted with PicoGreenP TMP dye, which is a DNA intercalator that binds strongly to the double stranded DNA. Upon binding to DNA, PicoGreenP TMP dye fluoresces with an excitation of 485 nm and emission of 525 nm. The intensity of the fluorescent signal is proportional to the quantity of DNA in the standard or the samples.
![]() If you have published a paper by using any of our ELISA since 01/01/2023, kindly fill out the “Krishgen Publication Reward Application Form” with complete information and send it by at email: kbiinfo@krishgen.com, with the subject “Krishgen Publication Reward”. We will get back to you with the Amazon / Krishgen Credit Reward after we confirm it ASAP!
If you have published a paper by using any of our ELISA since 01/01/2023, kindly fill out the “Krishgen Publication Reward Application Form” with complete information and send it by at email: kbiinfo@krishgen.com, with the subject “Krishgen Publication Reward”. We will get back to you with the Amazon / Krishgen Credit Reward after we confirm it ASAP!