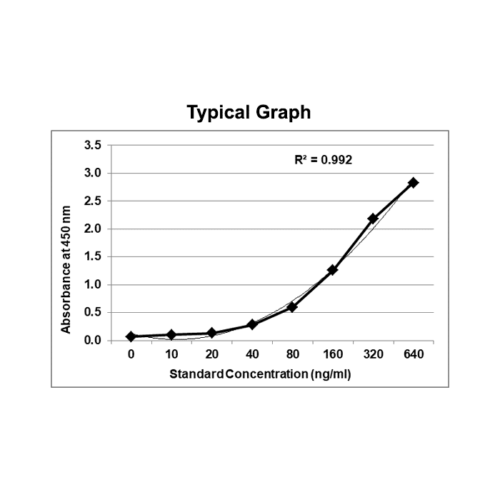
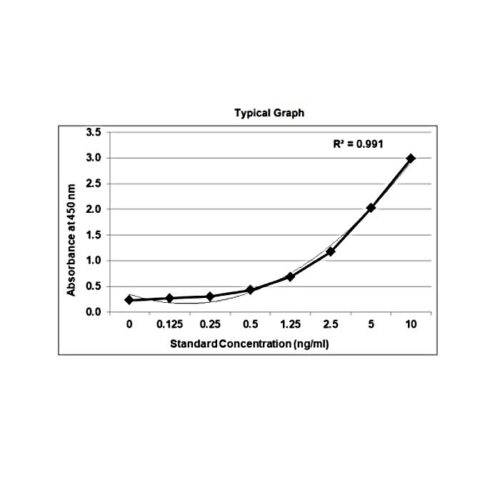
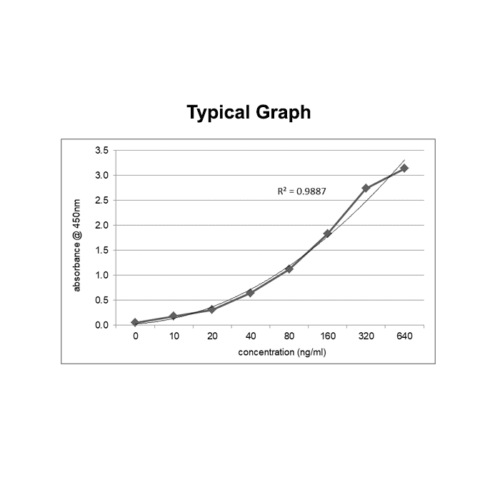
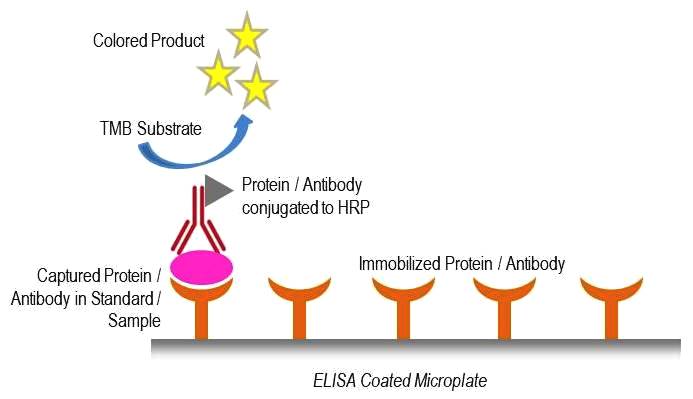
Therapeutic Drug Monitoring / mAb-based Biosimilars ELISA
KRIBIOLISA KRISHGEN BIOSYSTEMS offers Pharmacokinetics (PK) ELISA Kits for the quantification of serum and plasma protein drug levels to provide accurate pharmacokinetic (PK) data that will help optimize drug dosing regimens. These mAb-based ELISA are developed for use with biosimilars and biologics.
Our range of assays is currently the largest range of biosimilars ELISA in the world, with over 350 mAb drug targets covered for quantitative detection. Whether it’s development of biosimilar drugs or generics for monoclonal antibody drugs for cancer immunotherapy (Trastuzumab ELISA, Pertuzumab ELISA, Bevacizumab ELISA, Alemtuzumab ELISA etc), for auto-immune diseases like Crohn’s disease, multiple sclerosis, or rheumatoid arthritis (Infliximab ELISA, Natalizumab ELISA, Golimumab ELISA), or even for for ailments like osteoporosis or psoriasis (Ustekinumab ELISA, Denosumab ELISA), we have the right Therapeutic Drug Monitoring ELISA and Anti-Drug Antibody ELISA for you.
Several products are available with CE certification for clinical use, with others in the process of being approved. Please look for the “Regulatory Status” when choosing the assay (RUO or CE).
- All validation, verification and lot releases for these ELISA are performed according to best-in-class scientific guidelines – including a stringent seven-point validation to ensure quality and robustness lot-over-lot.
- Where available, validation of the kit against international standards from NIBSC / WHO
Learn more about our NIBSC validation on our blog here.
- These ELISA employ anti-idiotypic monoclonal antibodies in a sandwich assay format, ensuring exceptional specificity and sensitivity in drug detection, even at low concentrations.
- Calibrated against innovator drugs to ensure a high degree of accuracy
- To ensure no matrix interference and reproducible dilutional linearity, we run several serum and plasma spiking experiments at various dilutions to optimize. Inter and intra assay CV in accordance with FDA & EMEA requirements CV <10%.
Pool Intra Assay %CV Inter Assay %CV Low <10% <10% Medium <5% <5% High <5% <5%
- Validated against ICH / EMA guidelines under the ISO 13485 certification.
- 96 well plates with break apart strips includes lyophilized standards for stability.
- Expiry period of a minimum 10 months
- Ship at ambient temperature, with lyophilized / stabilized reagents
Performance Characteristics and Validation Information:
Sensitivity: The limit of detection is defined as the lowest detectable concentration corresponding to a signal of Mean of ‘0’ standard plus 2* SD. Each kit mentions sensitivity in the datasheet.
Calibration: Several KRIBIOLISA kits have been Calibrated against International Standards from the National Institute of Biologicals and Control (NIBSC), Potters Bar, Hertfordshire EN6 3QG, UK.
The Standards provided in the kit are also calibrated against commercially sourced drugs and alternate recombinant biosimilars.
Recovery and Matrix Effect:

Precision: Precision is defined as the percent coefficient of variation (%CV) i.e. standard deviation divided by the mean and multiplied by 100. Assay precision was determined by both intra and inter assay reproducibility on two pools with low, medium, and high concentrations. While actual precision may vary from laboratory to laboratory and technician to technician, it is recommended that all operators achieve precision below these design goals before reporting results.
360+ Pharmacokinetic (PK) ELISA
Krishgen manufactures the world’s largest range of ELISA for biosimilars research and therapeutic drug monitoring. Need a custom assay range or want to run an animal sample? Speak to our team today!
Featured Citations
Related Research Areas
Featured Products
Related Technical Notes
Therapeutic Drug Monitoring / mAb-based Biosimilars ELISA
As biosimilars continue to gain prominence in healthcare, it becomes essential to monitor their concentration levels in patients receiving treatment. These complex biological drugs, derived from living organisms, require thorough analysis to ensure consistent quality, efficacy, and safety
About Pharmacokinetics:
Pharmacokinetics (PK) is the study of how a drug is absorbed, distributed, metabolized, and excreted over time. Understanding the pharmacokinetic and pharmacodynamic behavior of a given therapeutic drug is an essential element of understanding its effectiveness and safety, as well as identifying the proper dosage and distribution. mAb-based bioanalytical testing methods for pharmacokinetic analysis are used to determine concentration time profiles of the drug and metabolites in biological sample fluids, providing information necessary for PK analysis. mAb-based biosimilars assays are a vital component of the drug development process, and the data derived is used to help select dosage for preclinical and clinical studies. PK studies are also essential in designing combination drugs and re-purposing existing drugs for new therapies.
About Therapeutic Drug Monitoring:
Therapeutic Drug Monitoring is the clinical practice of measuring drugs at designed regular intervals in an on-going treatment. This is done by clinicians in order to maintain and monitor the in vivo concentration in a patient in order to optimise their individual dosage regimen. This is particularly essential for drugs that have narrow therapeutic ranges, are marked with pharmacokinetic variability or drugs that are known to cause therapeutic and adverse effects.
KRIBIOLISA mAb-based ELISA Kits:
Krishgen’s range of ELISA for pharmacokinetic and anti-drug antibody (ADA) quantification allow both clinicians and researchers to measure pharmacokinetic characteristics of the drug accurately and reliably.
Popular Products by Research Area:
Anti Cancer
[product_table id="18637"]
Checkpoint Inhibitors
[product_table id="18642"]
Anti-Inflammatory
[product_table id="18643"]
Anti-TNFα
[product_table id="18644"]
Wet AMD
[product_table id="18645"]
Anti-Allergy and Asthma
[product_table id="18652"]
Immune Stimulation
NA
Obesity
[product_table id="18653"]
Osteoporosis
[product_table id="18655"]